|
|
IBBJ
Winter 2015, Vol 1, Issue 1
Injectable Estradiol Valerate, as a Substitute for Estradiol Pellets in Breast Cancer Animal Model
Ramezan Behzadi1, Sadegh Fattahi1, Mohammad Reza Momtaz1, Saeid Kavoosian1, Mohsen Asouri1, Haleh Akhavan-Niaki2*
1- North research Center-Pasteur Institute of Iran, Amol, Iran
2- Department of Anatomy, Babol University of Medical Sciences, Babol, Iran
Submitted 5 Feb 2015; Accepted 8 Feb 2015; Published 10 Feb 2015
The ability to maintain and study human tissues in in-vivo environment has proved to be a valuable tool in breast cancer research for several decades. The most widely tissues have been xenografts established human breast cancer cell lines into athymic nude mice. The aim of this study was to provide a new accurate and affordable method for the establishment of breast cancer xenograftin nude mice. Injectable estradiol valerate was assayed as a substitute for estradiol pellets that are rather expensive in order to create cancer tumors by MCF7 xenograft method in nude mice (B6). Twenty four healthy adult female nude mice (B6) were injected with different concentrations of pre-counted MCF7 cells. Then estradiol valerate and Matrigel B.D. were injected either alone or combined in two different groups of animals. In a period of 6 to 9 weeks, mice injected with increased amount of MCF7 cells, estradiol valerate and Matrigel (combined or alone) developed faster and larger tumors than animals which received MCF7 only or MCF7 and Matrigel combined. The results indicate that estradiol valerate which is way less expensive than estradiol pellets can be used as tumor proliferator to create animal breast cancer models.
Key Words: Etradiol valerate, nude mice, breast cancer, MCF-7
|
|
* Corresponding author: Haleh Akhavan-Niaki
Tell: +98 9111255920
E-mail: halehakhavan@yahoo.com
|
|
|
B |
reast cancer is the most leading type of cancer among women throughout the World (1). Current breast cancer chemotherapies, however, have not been satisfactory and researchers are constantly looking for newer protocols. Recently in-vitro studies on animal or human cell lines have led to introduce successful methods that can be helpful in pilot studies in cancer research (2-5). Ultimately, animal models such as mice are used to initiate an in-vivo breast tumor xenograft in mice in which their defense system are primarily destroyed (6-9). MCF7 cells are commonly used as a source of human breast cancerous cells. As most MCF7 cell lines possess estrogen receptor (ER+), estrogen is usually administrated to help tumor growth (7, 10). Estrogen pellets are often used as a source of estrogen (11-12). The estrogen pellets, however, are rather expensive and most research laboratories cannot afford them. Although some researchers have been tried less expensive agents namely estradiol cypionate (7) as an alternate to estrogen pellets. In this study, we used injectable estradiol valerate as a tumor proliferator and investigated its effect on tumor size and proliferation rate as well as its pathogenic feature.
��� Materials & Methods �������� �������������������������
Materigel was purchased from BD Biosciences (BD Biosciences, Belgium). Injectable estradiol valerate was purchased (Aburaihan Pharmaceuticals Co, Iran).
Cell line:
MCF7 cell line was obtained from Pasteur institute of Iran, Tehran, Iran. The cells were cultured in RPMI 1640 medium (PAA, Austria) supplemented with 10% FBS and 1% antibiotics penicillin/streptomycin (Invitrogen, USA) at 37�C in an incubator with humidified atmosphere containing 5% CO2 and 95% air. After reaching 80- 90% confluence, cells were briefly trypsinized by addition of 0.05% trypsin and centrifuged at 1500 rpm for 5 min, and the pellet was refrigerated for further use.
Animals:
Twenty four healthy adult female nude mice (B6) aged 7 to 9 weeks and weighting 20 to 25 g were obtained from North Research Center, Pasteur Institute of Iran. The animals were housed in polypropylene cages and maintained under standard conditions (12 h light: 12 h dark cycle; 25 � 30�C; 35�60% humidity).
Tumor induction:
Normal healthy nude mice were randomly divided into four groups of six as indicated in Table 1. All mice were intramuscularly (IM) injected with different concentrations of pre-counted cells (5, 10, 20�106 cells) in their right thighs. Estradiol valerate (equaling to 1 mg/kg estradiol valerate) was IM injected into mice two days prior to MCF7 cells injection and once weekly. Other groups received either matrigel or a combination of estradiol valerate and matrigel, prior to MCF7 cells injection. A negative control group was injected with MCF7 cells without prior treatment. Table 1 represents the injection program of MCF 7 cells into each nude mice group.
Pathological Studies:
Selected mice from different groups were sacrificed and their representative sections of tumor with adjacent tissues were fixed in 10% neutral buffered formalin for 24 to 36 hours. Paraffin-embedded sections were prepared with 4μm thickness followed by standard H & E staining. Prepared tissues were examined using a light microscope.
Table 1: MCF7 cell injection program
|
Other Injectable Materials |
MCF7 Cell Counts (�106) |
Groups |
|
|
estradiol valerate 2mg/kg |
5, 10, 20 |
I |
|
|
- |
5, 10, 20 |
II |
|
|
Matrigel |
5, 10, 20 |
III |
|
|
estradiol valerate 2mg/kg and matrigel |
5, 10, 20 |
IV |
|
��� Results ������������ ��������� ��������� ��������������������������
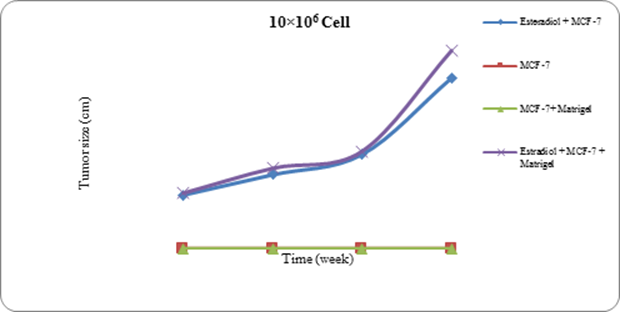
Mice who were not stimulated with estradiol valerate prior to MCF7 cells injection (groups II and III) did not develop any tumor. In other groups (I & IV) tumors sized 2 mm were observed during 6 to 9 weeks post injection (Figure 1) and all mice developed tumors. In group IV which received 300 �l matrigel, compared to group I, 2mm sized tumors appeared more rapidly. However, in both groups the time of tumor development decreased with the increase of the number of injected MCF7 cells while the rate of tumor growth increased. Figures 2 to 4 represent the kinetic of tumor development in different mice groups receiving 5, 10, 20�106 cells respectively.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pathological observations:
In examination of prepared tissues, tumoral lesions with wide necroses consisting of cells with large nucleus and course chromatins were observed. Most of the cells were vesicular and many possessed nucleolus and/or convex nucleolus. Minimal cytoplasm with indefinite boundaries which basically were accumulated or in a form of cellular disks was observed (Figure 5).
|
|
|
|
|
|
��� Discussion� ��������������� ��������� �������������������������
Human breast tumor xenografts provide the opportunity to study various important interactions between the tumor and host tissues, including endocrinologic, immunologic and tumor-stroma interactions. Our data clearly demonstrate that no tumor growth can occur in the absence of estradiol injection as estradiol is a potent tumor prolifator. However, the concomitant injection of estradiol and matrigel can accelerate tumor's formation without influencing its size or progression rate after reaching 2 mm, confirming the fact that materiel has the ability to enhance tumor growth [13]. This suggests that stimulating agents or nutrients present in matrigel may help cell growth but are not sufficient by themselves in this animal model. Also, the initial amount of injected MCF7 cells influences the size of tumor.
Based on the findings in this study it can be concluded that the use of injectable estradiol valerate, can be substituted to other forms of estrogen as a tumor promoter reducing expenses in cancer research.
Conflict of interests:
Authors declare no conflict of interest.
��� References����������������� ��������� � �����������������������
1. DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011 Nov-Dec;61(6):409-18.
2. Fattahi S, Ardekani AM, Zabihi E, Abedian Z, Mostafazadeh A, Pourbagher R, et al. Antioxidant and apoptotic effects of an aqueous extract of Urtica dioica on the MCF-7 human breast cancer cell line. Asian Pac J Cancer Prev. 2013;14(9):5317-23.
3. Fattahi S, Zabihi E, Abedian Z, Pourbagher R, Motevalizadeh Ardekani A, Mostafazadeh A, et al. Total Phenolic and Flavonoid Contents of Aqueous Extract of Stinging Nettle and In Vitro Antiproliferative Effect on Hela and BT-474 Cell Lines. Int J Mol Cell Med. 2014 Spring;3(2):102-7.
4. Moreno J, Krishnan AV, Feldman D. Molecular mechanisms mediating the anti-proliferative effects of Vitamin D in prostate cancer. J Steroid Biochem Mol Biol. 2005 Oct;97(1-2):31-6.
5. Movsesyan VA, Stoica BA, Yakovlev AG, Knoblach SM, Lea PMt, Cernak I, et al. Anandamide-induced cell death in primary neuronal cultures: role of calpain and caspase pathways. Cell Death Differ. 2004 Oct;11(10):1121-32.
6. Clarke R. Human breast cancer cell line xenografts as models of breast cancer. The immunobiologies of recipient mice and the characteristics of several tumorigenic cell lines. Breast Cancer Res Treat. 1996;39(1):69-86.
7. Johnson CH, Manna SK, Krausz KW, Bonzo JA, Divelbiss RD, Hollingshead MG, et al. Global metabolomics reveals urinary biomarkers of breast cancer in a mcf-7 xenograft mouse model. Metabolites. 2013;3(3):658-72.
8. Weber Lozada K, Keri RA. Bisphenol A increases mammary cancer risk in two distinct mouse models of breast cancer. Biol Reprod. 2011 Sep;85(3):490-7.
9. VanWeelden K, Flanagan L, Binderup L, Tenniswood M, Welsh J. Apoptotic regression of MCF-7 xenografts in nude mice treated with the vitamin D3 analog, EB1089. Endocrinology. 1998 Apr;139(4):2102-10.
10. Dabrosin C, Palmer K, Muller WJ, Gauldie J. Estradiol promotes growth and angiogenesis in polyoma middle T transgenic mouse mammary tumor explants. Breast Cancer Res Treat. 2003 Mar;78(1):1-6.
11. Lindberg MK, Weihua Z, Andersson N, Moverare S, Gao H, Vidal O, et al. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol. 2002 Aug;174(2):167-78.
12. Robertson FM, Mallery SR, Bergdall-Costell VK, Cheng M, Pei P, Prosperi JR, et al. Cyclooxygenase-2 directly induces MCF-7 breast tumor cells to develop into exponentially growing, highly angiogenic and regionally invasive human ductal carcinoma xenografts. Anticancer Res. 2007 Mar-Apr;27(2):719-27.